The Insider has already told the story of the development and introduction to the Russian market of favipiravir, the ineffectiveness of which in COVID-19 has been proven by foreign studies. Another drug from the same manufacturer, Elpida (elsulfavirin), entered the market almost as quickly, is also not used in other countries of the world and raised no less questions from patient organizations. At the same time, it costs more than the internationally recognized and thoroughly researched drug Tivicay (dolutegravir), instead of which it is purchased. In less than five years, the state has purchased Elpida for patients for about 7 billion rubles.
One of the explanations for the unprecedented success of the drug may be that Alexei Mazus, the chief specialist of the Russian Ministry of Health on HIV infection, is associated with the manufacturers of the drug (The Insider wrote about how the Ministry of Health failed to fight HIV under his leadership).
Mazus does not believe in condom protection against HIV, denies the need for treatment of drug addicts, and since the 1990s has promoted the idea that mass forced HIV testing will save Russians from infection. This wise policy has led to the growth of the HIV epidemic in Russia, some regions of the country are among the world leaders in the proportion of the adult population affected by HIV infection, and only half of all patients are provided with the necessary drugs. But the more HIV carriers there are and the greater the shortage of drugs recognized by international standards, the more expensive and in large volumes one can sell one's know-how, the Elpida miracle drug. Meanwhile, how well this drug works and how it is combined with other drugs is essentially unknown – the manufacturer managed to register Elpida with only a couple of small clinical studies with a strange design. By recommending it, the Ministry of Health is essentially experimenting on the health of terminally ill patients while bringing money to the accounts of a friendly pharmaceutical business.
"Elpida" – a small miracle or a dummy
The history of Elpida's manufacturer, Khimrar, was presented in the media in the mid-2000s as one of the clearest examples of the development of innovations and the attraction of foreign investment to Russia. At the end of 2004, she opened a research center in the field of preclinical drug development in Khimki near Moscow. The investor was the American fund Torrey Pines Investment (actually not so American, but more on that later). $ 5 million was invested in the center, he began to research new substances that could potentially become medicines. The customers of these studies were pharmaceutical companies – such a scheme is regularly used in the world, as it saves money for large pharmaceutical corporations. The acquaintances in the Ministry of Industry and Trade also helped to advance the business – the founders of the company have long been friends with Sergey Tsyb, who in those years headed the department of the ministry responsible for pharmaceuticals.
However, the founders of the company dreamed of not just fulfilling other people's orders for the synthesis and verification of molecules. “I want to create my own medicine and make money as it should,” Andrey Ivashchenko, the founder of the company, said in an interview with Forbes in 2009. At the same time, Himrar began to take steps in this direction and established the Viriom company.
Immediately after its founding, Viriom entered into a contract with the Swiss F. Hoffman-La Roche Ltd to research several molecules that had been developed at La Roche since the early 2000s. The partners felt that the new molecules could be effective against chronic viral infections: HIV and hepatitis B and C. La Roche decided to stop research on them, as it focused on anticancer drugs. In Russia, where in 2009 there were already about half a million HIV-infected people (only official ones, with an established diagnosis), they became interested in promising molecules. At the same time, the stage at which the developments of the Swiss company were located was not even clinical trials. A year later, from the received package of molecules, Viriom chose the one that began to be tested as a cure for HIV. She received the working title VM-1500 and eventually became the very "Elpida".
The project was supported by the Commission for Modernization and Technological Development under President Dmitry Medvedev. In 2011-2013, Viriom received 150 million rubles from the Ministry of Industry and Trade for the development and research of the drug. Already in 2013, the company stated that elsulfavirin has a pronounced antiviral effect observed in patients with HIV. To the sharp question of a Kommersant journalist: “Is this really our long-awaited breakthrough in the most modern industry – pharmacology – and the challenge of the global AIDS epidemic in the world ?!” co-owner of Khimrar, university comrade Ivashchenko Nikolai Savchuk answered : “The creation of a truly breakthrough new drug is almost always a small miracle.”
This miracle, however, was poorly supported by clinical trials. According to open data from clinical trial registries, the company conducted only three human studies until 2013. The number of volunteers did not exceed a few dozen, almost all of them were healthy, and they checked the pharmacokinetics of the substance – how the substance is converted into others while in the body and how it is then excreted from the body. During a seven-day study in Thailand, 14 HIV-infected patients received the drug for seven days, after which the researchers concluded that the drug showed "optimal antiviral activity." This was the only placebo-controlled trial of Elpida – that is, one group of patients was given a "dummy", the other was given a new medicine. And the number of study participants was very small. “Such a sample is only suitable for a preliminary study, that is, to determine whether there is any signal in terms of effectiveness at all. Seven days is also not enough time to evaluate the effectiveness. Typical samples used to study effectiveness in HIV are several hundred people,” Ilya Yasny, head of scientific expertise at the pharmaceutical venture fund Inbio Ventures, told The Insider. For example, the US Food and Drug Administration (FDA), which is authoritative for doctors all over the world, notes that several hundred people should take part in this phase of drug efficacy research. This is important not only to avoid statistical error, but also, in the case of HIV, to understand how the drug works in patients with different health conditions or virus mutations.
The first study on a relatively large group began in Russia in 2014 and lasted almost two years. The scientists compared the effectiveness of Elpida (in a three-drug regimen) with efavirenz (Stokrin, along with two similar drugs). Efavirenz was then the first-line treatment (i.e., the preferred treatment if there were no contraindications) in developed countries, but patients often refused it due to a large number of side effects, including psychiatric (depression, nightmares, insomnia, confusion consciousness, etc.). The authors of the Russian study stated that such side effects were less common in those who took Elpida than in those who took Stokrin. But there were no side effects, for example, EpiVac also had a medicine for COVID-19 simply because it was a pacifier . Did Elpida manage to show any efficiency?
In short, the very design of the study was such that no conclusions can be drawn from it that Elpida is at least in any way effective. Let's start with the methodology. In the published article, the authors refer to the design of the study as “multicenter, randomized, partially blind, direct comparison.” If translated from scientific language to ordinary language, this means that the trials were conducted in several hospitals (multicenter), scientists and patients did not know who was participating in which part of the experiment (randomized), some participants in the experiment (most likely doctors and / or manufacturers) knew about which participants are taking which medications (partially blinded), the study compared multiple drugs (comparative) and expected that the new drug would improve patients' quality of life (direct). In fact, the authors pointed out that a previous study in 14 patients already proved that the drug lowered the viral load well, and they were only interested in how much easier it was for patients to tolerate than Stokrin.
“This article <in the scientific journal – The Insider> violates many of the CONSORT rules that dictate how a randomized trial should be described. It is not clear how randomization was carried out, why it is partially blind, where the drugs come from. The most important violation – it is not said what is the final primary criterion. You must first formulate a hypothesis, and then test it statistically,” notes Ilya Yasny. According to the expert, such a study would not be sufficient for the drug to be registered in “normal jurisdictions”. In addition, modern GMP and GCP standards, good manufacturing and clinical practice are not fully observed in Russia. This can lead to the fact that manufactured drugs may be of poor quality, and research data may be falsified in whole or in part. Ilya Yasny points out that the researchers changed the end criteria after the end of the study. If at first they set out to test the activity of a drug for suppressing the virus, then at the end they indicated only a comparison of the activity with Stokrin, without even indicating how this effectiveness would be measured, and how to determine which drug is better.
The authors of the trials state that both treatment regimens (with Elpida and with Stokrin) showed approximately the same effectiveness, but the presented study results cannot be considered reliable, Yasny points out, due to the fact that there are errors in the design of the study, the results are poorly described. . And even more so, in no case can a drug be considered worthy of a recommendation based on the result of one such study.
The Ministry of Health did not think so, and based on the results of this one study, it issued a license to Elpida (and the regional health departments immediately began to purchase the medicine), and a few months later recommended that it be included in the first – that is, preferred – line of HIV therapy. The drug immediately began to buy AIDS centers throughout Russia. In fact, the drug had previously been studied in less than 200 people. The results of such studies by world standards are not reliable enough to start prescribing the drug to all patients in a row. Comparison of the efficacy and tolerability of Elpida with other drugs, except for efavirenz, was also not carried out – probably because, according to Russian clinical guidelines , and according to WHO recommendations of those years, it was efavirenz that was included in the preferred first line of therapy.
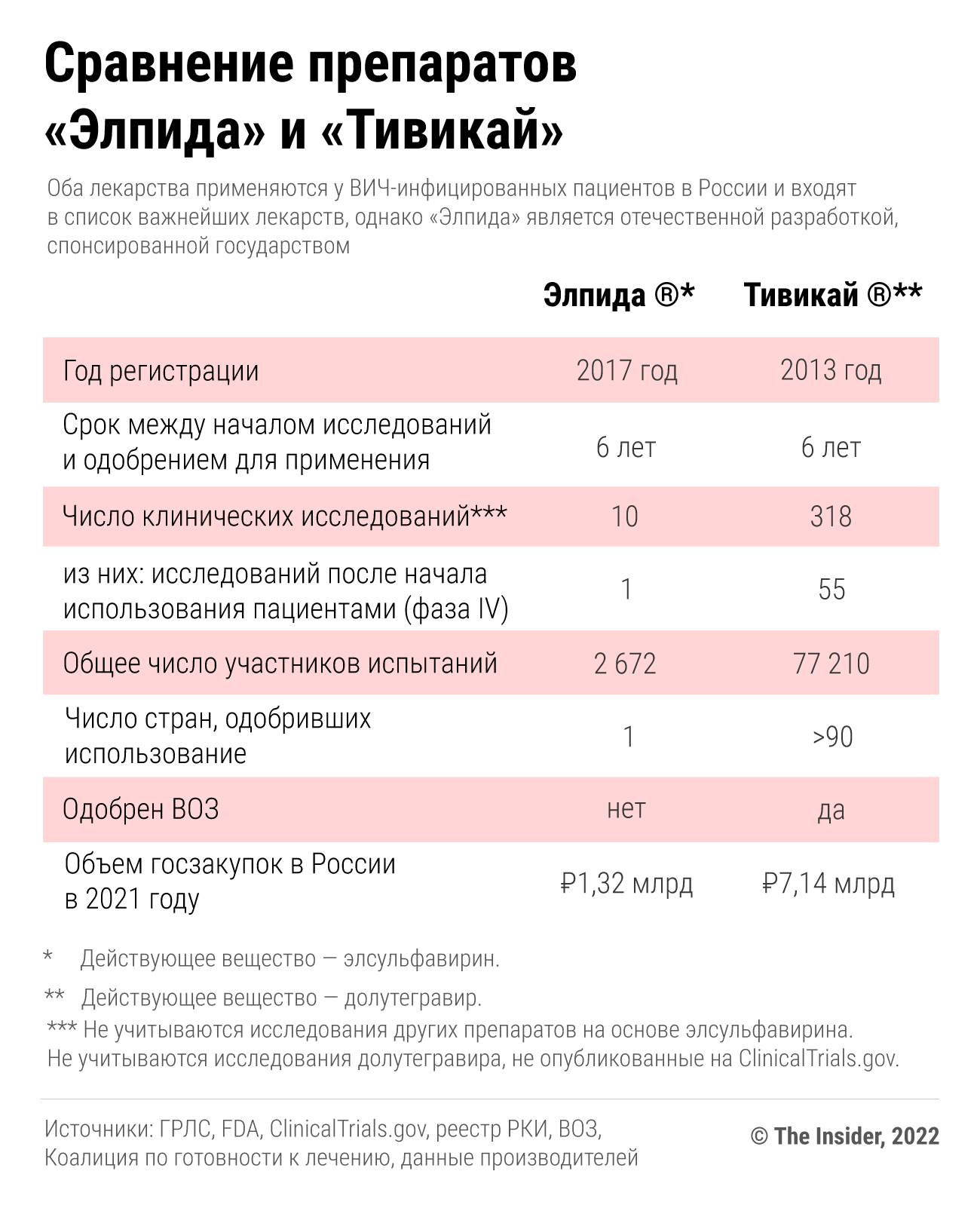
The noisy success of Elpida in Russia was not noticed outside of it, in 2019 WHO recommended that a completely different drug, dolutegravir (Tivicay), be included in the first line of therapy, as it turned out to be more tolerable and effective than other drugs of the same group ( The European AIDS Clinical Society (EACS) suggested doing this back in 2017).
At first (in 2019), Elpida (elsulfavirin) appeared in the draft clinical guidelines for the treatment of HIV in a rather modest role: the drug was recommended only as an alternative therapy for people who are not suitable for the most prescribed and preferred drug in the regimen for HIV patients, efavirenz. In addition, the first drafters of the recommendations at first honestly mentioned that Elpida should not be used in a number of cases, for example, in combination with the main drugs for tuberculosis – rifabutin and rifampicin, or with omeprazole (the main drug for gastritis), with some female contraceptives ( "Rigevidon"), the antibiotic clarithromycin – in a word, in combination with a number of fairly popular drugs.
However, already in 2020, Evgeny Voronin, the chief freelance HIV specialist of the Ministry of Health (who wrote these recommendations), was replaced by Alexander Mazus, who returned to this position, and immediately, with his participation, new recommendations were adopted, where Elpida was not only included in the preferred treatment regimen, but all information on interactions with other drugs was removed from the recommendations. In fact, if the doctor followed these recommendations and did not read the instructions for the drug (which describes dozens of drugs that cannot be used with Elpida or can be used, but with great care), he could prescribe a regimen to the patient, which would eventually cause harm health.
The new recommendations, including Elpida in the first line of therapy, do not deny that the drug is almost not studied: the schemes with its participation are marked with a 4C confidence level – according to the Ministry of Health, this stands for "weak recommendation (lack of evidence of adequate quality)" . However, in the end, this does not prevent advising to start therapy with this particular drug.
Cabal between officials and manufacturers
Simultaneously with the return of Mazus, the non-profit National Virological Association also became active. In 2021, the association received more than 19 million rubles in profit from commercial activities – this has not happened in the previous seven years of its history.
Honorary members of this organization include Anita Smith and William Shepard Smith Jr. Christian activists who have received US budget support to fight HIV in children since the days of President George W. Bush. Their funding from the government raises questions from other organizations, as they stigmatize risk groups (homosexuals and drug users), oppose the use of condoms – in general, they do everything that Mazus and his associate Lyudmila Stebenkova do in Russia (and for public money). ), although such practices around the world have shown to be ineffective in the fight against HIV.
It is noteworthy that at least seven foreigners are listed on the website among the honorary members of the Mazus Association, but the organization has never reported on receiving funds, for example, membership fees, from foreign persons. But it is even more interesting that after the restart of the association's website, a section appeared there listing the member companies of the organization. It turned out that over the years of their existence, there have been as many as two of them: Janssen (pharmaceutical division of Johnson & Johnson) and Viriom, the manufacturer of the same Elpida, which is categorically recommended by the association for appointment in the first line of therapy, despite the fact that this is one of the most expensive medicines available in Russia today. In fact, Mazus did not even hide the fact that, using his official position, he included in the recommendations for the whole country a drug from the company that financed his organization.
Thus, the drug, the effectiveness of which was not proven by sufficient clinical trials according to international standards, was included in the list of recommended prescriptions in the first place, in the list of essential drugs. All this time, he was patronized by officials of the Ministry of Industry and Trade, the “chief specialist” Aleksey Mazus, who receives a salary from the Ministry of Health, and large businessmen from the pharmaceutical industry. In 2022, the authorities purchased more than 55 thousand annual courses of the drug, spending 4.3 billion rubles. After Mazus returned to his position at the Ministry of Health, purchases of Elpida increased significantly – before that, it was bought for 8-11 thousand courses.
At the same time, patient organizations recorded a number of cases when, when taking Elpida, the viral load grew, that is, the drug did not suppress the reproduction of the virus. When the question about this was addressed to Viriom, the manufacturer noted that the matter may be in certain mutations of the virus, in which it becomes resistant to the drug. The fact is that the appointment of drugs of the same class as Elpida (non-nucleoside reverse transcriptase inhibitor, NNRTI) requires testing for mutations that cause drug resistance. However, since this analysis is expensive (more than 20 thousand rubles), in Russia it is almost never done at public expense, told The Insider in one of the patient organizations. The patients themselves may not be aware of the need for such an analysis, and if they do, not everyone can afford it. The company claims that Elpida is better than other drugs, as it is effective in many mutations where other drugs do not work. However, Viriom backs up its claims not with clinical trials of the drug, but with an analysis of the promising molecule RO-0335 carried out even before the sale to Himrar . How often patients have to end up canceling Elpida is hard to say – the Ministry of Health does not provide information about which drugs are often used by patients.
Questions to the manufacturer in patients also arise in connection with the rules for taking the drug. The fact is that antiviral therapy, as a rule, is very sensitive to the time of administration (you need to drink the medicine at the same hour every day) and to whether there was a recent meal. В инструкции к «Элпиде» эти данные неоднократно менялись, из-за чего пациенты не могли понять, как лучше принимать лекарство, чтобы от него точно был эффект. Такие манипуляции вызвали бурное обсуждение «сырости» лекарства на форумах ВИЧ-инфицированных.
Кто зарабатывает на «Элпиде»
День рождения «Вириома» — 30 апреля 2009 года — странным образом совпадает с появлением еще одной компании. В этот же день знакомый главного внештатного специалиста Минздрава России по ВИЧ-инфекции Алексея Мазуса бизнесмен Илья Цигельницкий зарегистрировал в Москве ООО «Медбиотест». После подписания контракта между «Вириомом» и швейцарской F. Hoffman-La Roche Ltd «Медбиотест» получил 5% в уставном капитале «Вириома», а немного позже, согласно ЕГРЮЛ, в капитал «Медбиотеста» — фактически, одного из бенефициаров новой разработки — вошла директор «АнтиВИЧ-Пресс» Александра Ускова (позже она поменяет фамилию на Мазус).
Дружба между Мазусом и «Химраром» на этом не закончилась. Сын Мазуса Матвей в 2018 году был зачислен на первый курс платной магистратуры МГИМО на программу «Государственно-частное партнерство». Обучение, судя по всему, прошло успешно, потому что уже через год «Химрар» передал ему треть в своей дочерней компании «Авивир», основной портфель которой с начала пандемии COVID-19 — перепродажа корейских тестов на антитела к вирусу. За неполных три года существования компания только напрямую продала российским больницам тестов более чем на 150 млн рублей.
После того как «Вириом» стал производить препарат «Элпида» для российских больниц, в его капитал стали входить представители крупных фармпроизводителей и люди, связанные с Минпромторгом. В 2018 году доли получили связанная с La Roche швейцарская компания APICON AG, Андрей Реус — бывший чиновник Минпромэнерго и глава «Оборонпрома», который с 2012 года, как и многие в Минпромторге, заинтересовался производством лекарств.
Еще один близкий к Минпромторгу человек, тогда же ставший совладельцем «Вириома», — Евгений Максимов. Этот предприниматель — основатель и руководитель Центра пластической и эндоскопической хирургии Натальи Мантуровой , супруги одного из самых богатых российских министров — Дениса Мантурова. Удивительно, но сам Максимов тогда сказал Vademecum, что не в курсе своего появления в капитале «Вириома», а потом перестал отвечать на звонки. Затем в 2019 году 30% акций «Вириома» перешли «Фармстандарту» миллиардера Виктора Харитонина.
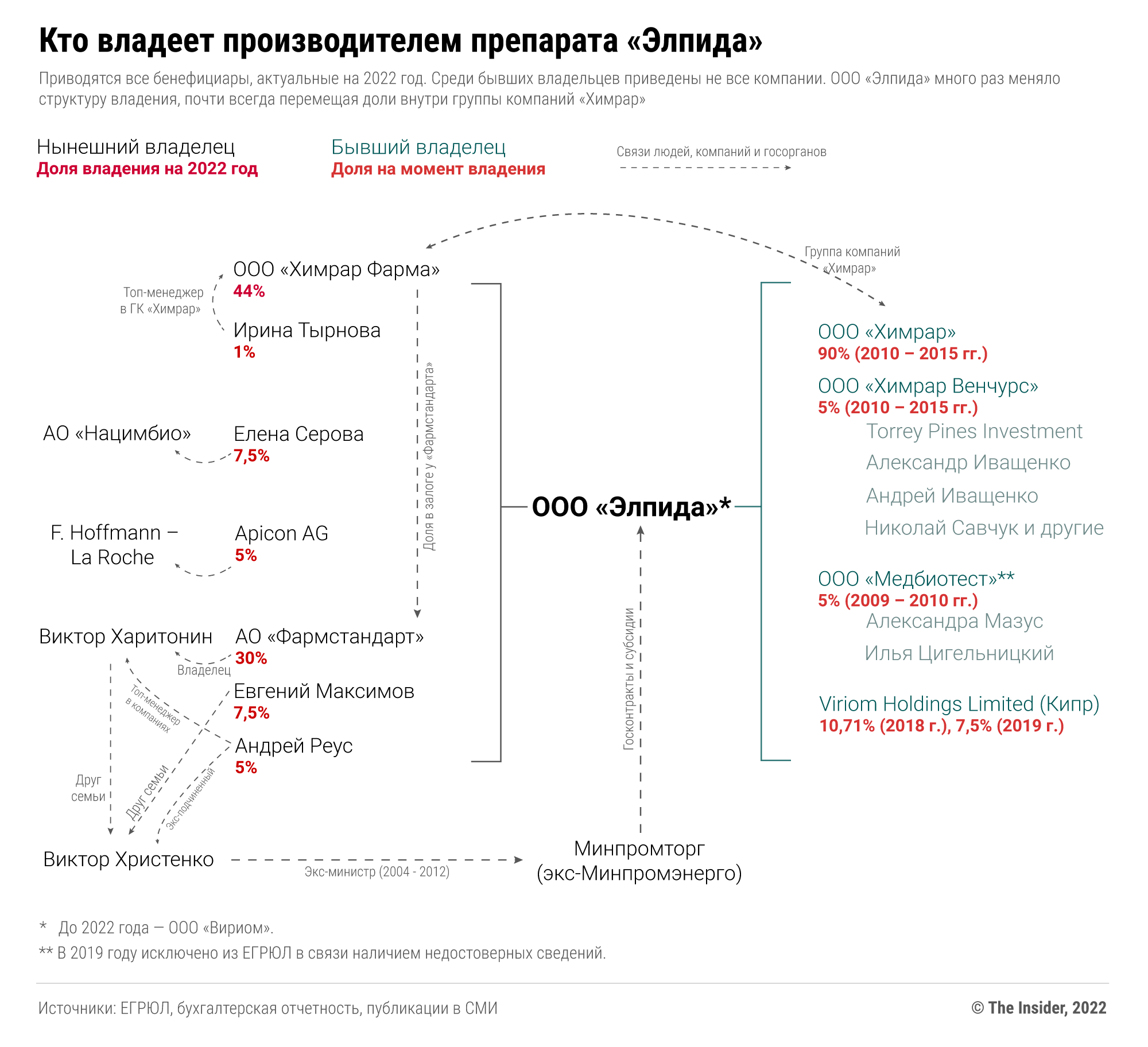
Таким образом, хотя продвижением лекарства «Элпида» и проведением исследований по нему занимается «Химрар», фактически производство и прибыли от его продажи по госконтрактам достаются в основном структурам Виктора Харитонина, а также людям, связанным с Минпромторгом.
Вложились сами в себя из Калифорнии: не очень иностранные инвестиции
В середине 2000-х «Химрар» приводили в пример как одну из высокотехнологичных компаний, привлекающих инвестиции из-за рубежа. Как мы помним, инвестором строительства центра в Химках стал американский фонд Torrey Pines Investment. На самом деле этот фонд всегда был связан с основателями «Химрара» — отцом и сыном Иващенко и Николаем Савчуком и их американским юрлицом ChemDiv. То есть, можно сказать, что привлеченные зарубежные инвестиции были получены у своего же бенефициара.
Вкладывать деньги в строительство в России основатели ChemDiv начали в 2002 году после того, как Владимир Путин утвердил документ «Основы политики Российской Федерации в области развития науки и технологий». Способствовать появлению в стране технопарков должен был Минпромторг, однако строительство центра обошлось без госинвестиций — для них в тот момент не существовало даже механизма. На базе построенного центра ученые продолжили заниматься созданием коллекции химических соединений (химики называют их библиотеками) — через несколько лет, по заявлению самих основателей «Химрара», их коллекция будет самой большой в мире. Фактически это означает, что химики компании создали сотни тысяч разных молекул и технологий их синтеза — потом такие молекулы можно опробовать для разных «мишеней», чтобы создавать лекарства. Для следующего этапа нужны инвестиции, но в то время в России биотехнологии еще не были популярны. Основатель «Химрара» Андрей Иващенко сетовал на то, что частные фонды не готовы вкладываться в фармацевтику, и призывал государство заняться этим:
«Сегодня академические исследования в России доводятся до определенного этапа, условно говоря, — до первого успеха в пробирке. Но дальше их никто не подхватывает, отечественная индустрия готова, как правило, брать продукты, прошедшие третью фазу клинических испытаний. Между этими двумя этапами — пустота в шесть-семь лет и несколько десятков миллионов долларов по каждому продукту. Поэтому задача первая — замкнуть цикл, и для этого есть только один путь: государство должно взять на себя финансирование “промежуточной” фазы».
Государство эти призывы услышало и в 2011 году приняло федеральную целевую программу «Фарма-2020» — «Химрар» даже поучаствовал в ее разработке, а потом получил 13 контрактов с Минпромторгом на общую сумму около 1,9 млрд рублей. Именно по этой программе его дочка «Вириом» получала инвестиции на разработку «Элпиды», которая фактически остается одним из двух успешных препаратов в портфеле «Химрара». Второй — «Авифавир» (фавипиравир), напомним, был разработан в первые же месяцы эпидемии COVID-19 под покровительством Российского фонда прямых инвестиций, Минпромторга и Фонда содействия инновациям. Как и «Элпида», препарат получил регистрационное удостоверение и госконтракты на сотни миллионов рублей, практически не проходя клинических испытаний.
Зарегистрировав «Элпиду» и заручившись поддержкой в виде топ-менеджеров Минпромторга и «Фармстандарта», основатели «Вириома» решили пропиарить ее в США. Николай Савчук и другие топ-менеджеры, связанные с американской компанией ChemDiv, основали в Сан-Диего, где находится офис ChemDiv, новую компанию — Viriom Inc. Компания утверждает , что специализируется на разработке антивирусных лекарств, но фактически в ее портфеле — только «Элпида» и ее комбинация с тенофовиром и эмтрицитабином, зарегистрированными в России и не прошедшими достоверных клинических исследований, а также тот самый фавипиравир для лечения COVID-19.
На сайте американского Viriom Inc. не упоминается ни одна аффилиация с российским «старшим братом». Однако даже логотип компании совпадает с тем, который российский «Вириом» годами использовал, представляя результаты своих исследований на международных конференциях.

Американская база данных компаний Crunchbase указывает , что в состав совета директоров Viriom Inc. входит и Алексей Мазус, а также что он является ее советником. Источник этой информации неизвестен, однако единственное масштабное исследование «Элпиды» на инфицированных добровольцах в России проводилось как раз в инфекционной больнице №2 на Соколиной Горе в Москве, где находится СПИД-центр Мазуса.
Летом 2022 года Viriom Inc. также добавила в портфель депулфавирин — инъекционный препарат против ВИЧ, который предполагается использовать для редкого (раз в месяц) использования. Хотя Viriom пишет, что он уже был одобрен для вывода на рынок, ни в России, ни в других странах он неизвестен. В июле 2022 года для продвижения препарата был создан сайт , однако по состоянию на сентябрь 2022 года на нем не было никакой информации. Судя по описанию препарата, речь идет о субстанции VM-1500A-LAI ( деселсуфавирин ), исследование которой проводилось в России в 2019–2020 годах. Результаты исследования фармакокинетики препарата в 2020 году на конференции в Бостоне представили сотрудники Viriom — и снова Алексей Мазус. Фактически это тот же элсульфавирин, однако в особенной инъекционной форме для применения не чаще раза в месяц. В апреле 2022 года в десяти российских больницах началось клиническое исследование эффективности и безопасности перехода на схему с этим препаратом на 505 ВИЧ-инфицированных добровольцах. Оно продлится полтора года. Это исследование проводит уже американская компания Viriom.
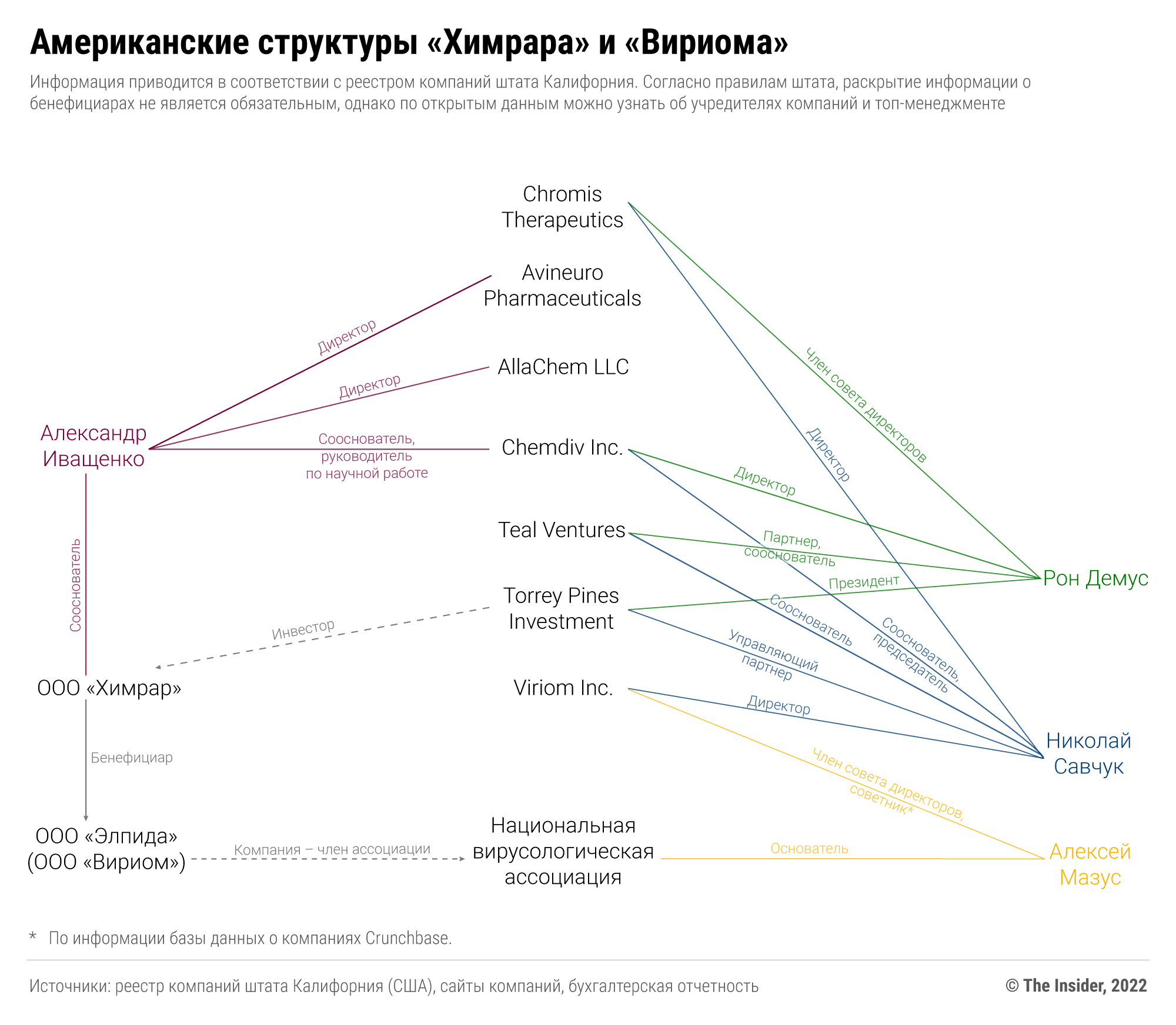
Таким образом, управляющими бизнеса «Химрара» в США, в том числе продвижения «Элпиды» как мировой инновации, является Николай Савчук, а также его американский партнер Рональд Демус, который работает в структурах Иващенко и Савчука уже 20 лет. Производитель «Элпиды» (кстати, в 2022 году российский «Вириом» переименовался в ООО «Элпида») и «Авифавира» имеет связи со «Сколково», РФПИ, Минпромторгом и другими российскими организациями, что не мешает Савчуку в своем профиле в профессиональной социальной сети LinkedIn осуждать российские власти за вторжение в Украину — в конце концов, он позиционирует себя в первую очередь американским предпринимателем, инвестором и ученым.
В работе над материалом использованы архивные публикации в российской прессе. Полные тексты этих публикаций, а также данные о клинических исследованиях «Элпиды», финансовые отчеты и учредительные документы организаций, упомянутых в расследовании, вы можете найти на Github . Автор благодарит экспертов, пожелавших остаться анонимными, за участие в подготовке материала. Для оформления статьи использованы иллюстрации, созданные с помощью нейросети DALL·E 2.


